
An alternative measure of composition is the convergence pressure of the system, which is defined as that pressure at which the K values for all the components in an isothermal mixture converge to unity. Based on Antoine equation, the boiling point for pure D is given by where A D, B D and C D are the Antoine coefficients for compound D. ( These will be used to generate initial guesses later on ). The Kellogg and DePriester charts and their subsequent extensions and generalizations use the molar average boiling points of the liquid and vapor phases to represent the composition effect. Solve for the boiling points of pure compounds. Determine boiling point temperature and composition of a vapor if pressure and liquid. 558.98 R (98.98 F) This gives a 1.03 deviation from DePriester chart. The boiling points are significantly different (-1☌ butane vs -43☌ propane), and this allows for an ok separation with the first stage - The chemical composition is very similar (both alkanes, only 1 CH2 different), and so the activity coefficient is close to 1, meaning close to ideal mixture behavior. Chapter 9 Practice Problems: 1 (use 8 MPa not 1.5, CO2 boiling point is 78.5C (194. The upper curve in the diagram is called the dew-point curve while the lower one is.

SI versions of these charts have been developed by Dadyburjor. These are the assumptions used to produce a DePriester Chart. This is a simple way of determining the boiling point of a pure compound at any. The vapor pressure and hence the boiling point of a liquid mixture.
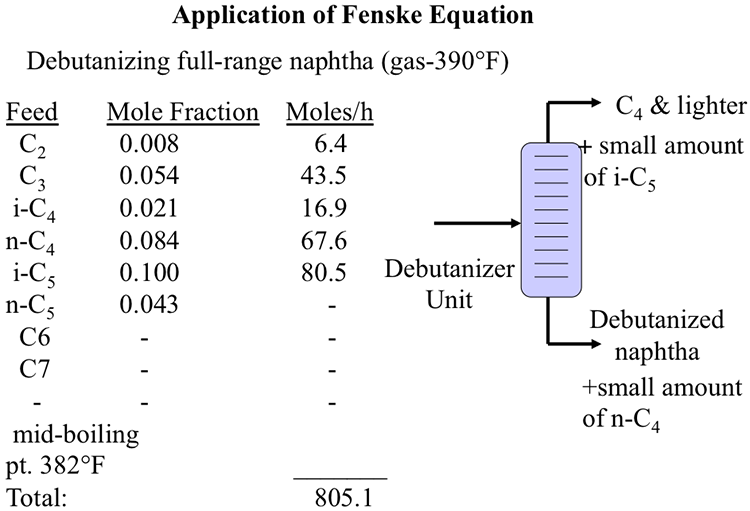
For K as a function of T and P only, the DePriester charts provide good starting values for the iteration. Calculations for these two limits are illustrated below. The limiting conditions of the two-phase region are the bubble point (100 liquid) and the dew point (100 vapor). If you reduce the pressure of column boiling point decreases and vaporisation increase resulting heavier components in top. One cannot calculate K values until phase compositions are known, and those cannot be known until the K values are available to calculate them. DePriester (1953) developed two nomographs which are of general use in the industry (Figs. Answer: Distillation column worked on the basis of boiling temperature or boiling point and boiling point depends upon pressure. The Kellogg charts, and hence the DePriester charts, are based primarily on the Benedict-Webb-Rubin equation of state, which can represent both the liquid and the vapor phases and can predict K values quite accurately when the equation constants are available for the components in question.Ī trial-and-error procedure is required with any K-value correlation that takes into account the effect of composition. These charts are a simplification of the Kellogg charts and include additional experimental data. The easiest to use are the DePriester charts, which cover 12 hydrocarbons (methane, ethylene, ethane, propylene, propane, isobutane, isobutylene, n-butane, isopentane, n-pentane, n-hexane, and n-heptane). Question DePriester Charts Finding Tboiling of the pure components: T boiling Ethylene -35.5 oC n-Pentane 153 oC n-Heptane >200 oC The boiling point. Also P-y1 curve has a maximum at the same point. 1 curve exhibits a maximum, when the positive departures from linearity become sufficiently large, relative to the difference between the two pure species vapor pressure. For example, several major graphical K-value correlations are available for light-hydrocarbon systems. 1 (or dew point curve) lies above the linear P-x.

However, for mixtures of compounds of similar molecular structure and size, the K value depends mainly on temperature and pressure. 4, the K value of a species is a complex function of temperature, pressure, and equilibrium vapor- and liquid-phase compositions.


 0 kommentar(er)
0 kommentar(er)
